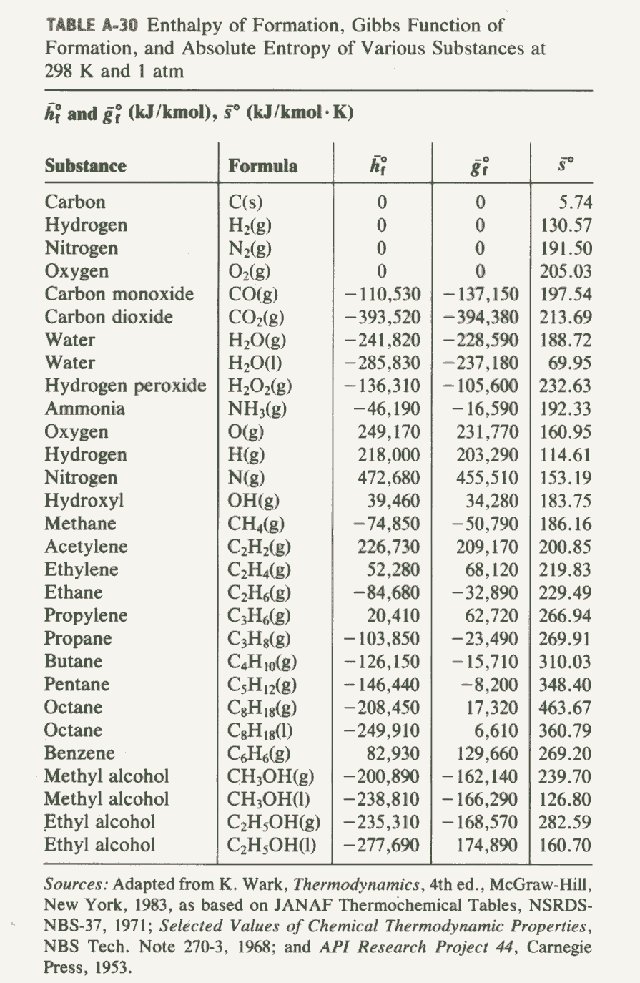
enthalpies of formation of the products minus the sum of the enthalpies

Standard molar capacities, enthalpies of formation , entropies.

Table: Experimental and calculated enthalpies of formation of elements (kcal
This information can be used to determine the relative enthalpy difference
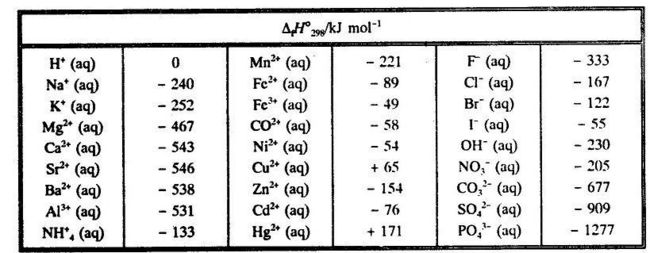
Conventional standard enthalpy of formation for ions in infinitely dilute

STANDARD HEAT OF FORMATION:

breaking up the lattice formation enthalpy in to numerous other steps.
Standard molar heat of formation of ZnBr2(c,l) from the elements,

Gravitational Potential Energy -> Kinetic Energy (in-fall) -> Heat

The engineer can now click along the plotted curve to determine enthalpy for

From this value and reaction 2, the CH3SO2 enthalpy of formation will be
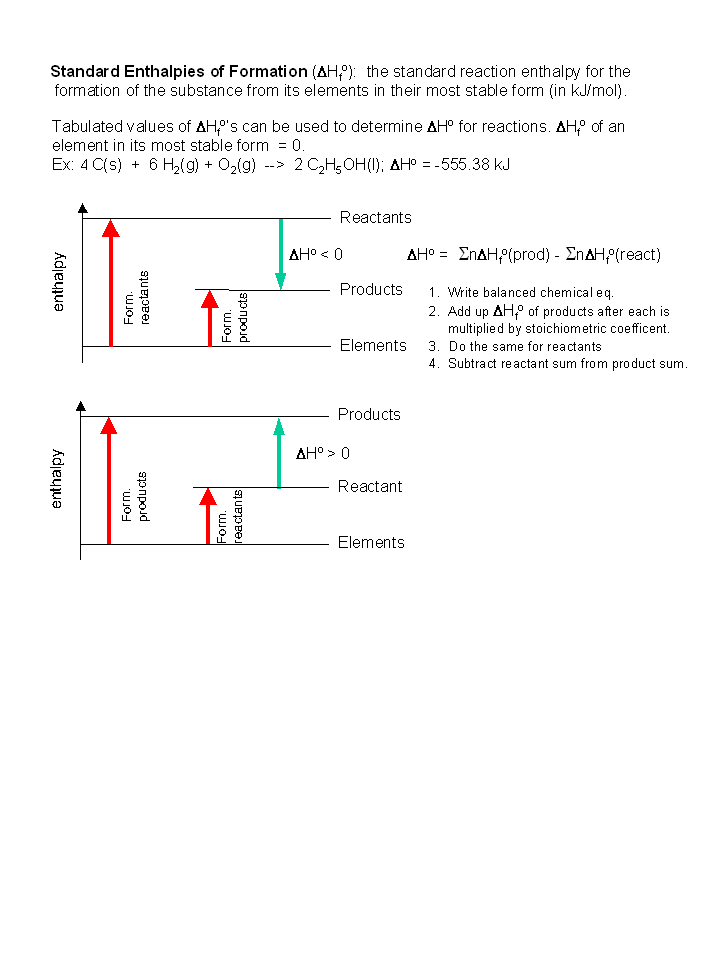
Standard Enthalpy of Formation
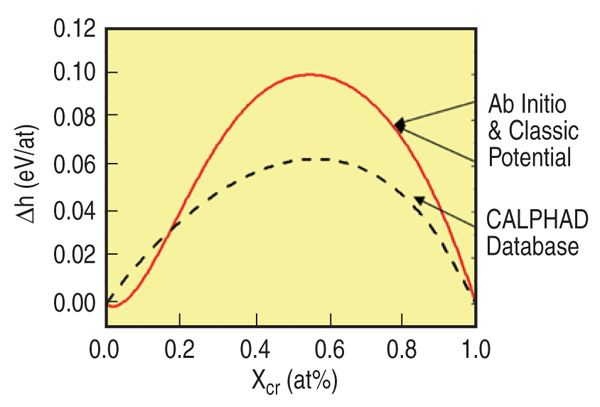
The heat of formation of a random solid solution of ferromagnetic FeCr as
The Chemical Origins of Reaction Enthalpy

Standard Enthalpies of Formation

The only other information you need, would be the "enthalpy of formation of

DHf - heat of formation in kcal mol-1 ; QN - net atomic charge on atom N;

Heat-induced formation of IgG complexes with dyes of different

A thermal component is associated with the leftover heat of formation (see

This process is similar to sweating in humans, effectively releasing heat
No comments:
Post a Comment